At New Jersey Precision Technologies, our mission is improving lives through manufacturing innovation. We are committed to becoming an integral part of your supply chain for medical device manufacturing by providing components with superior quality control and customer service from start to finish.
As an ISO 13485 certified company, New Jersey Precision Technologies, Inc is qualified to manufacture medical devices for a variety of applications, including surgical instruments and implants. Our quality management system meets the International Organization for Standardization’s (ISO) criteria for medical device manufacturing and affirms NJPT’s ability to meet our customers’ requirements as well as the latest regulatory requirements of the medical industry.
New Jersey Precision Technologies is a full-service contract manufacturer with more than 40 wire, sinker and CNC hole pop EDM machines, 20 CNC milling and turning centers, and a fully staffed engineering department to help you with your next project. Let us help you simplify your supply chain with fewer outside operations, lower costs and shorter lead times. We can handle any sized project from prototyping to production, including secondary operations, finishing, assembly and packaging, and Inventory and consignment programs are available to help you meet your customers’ needs.
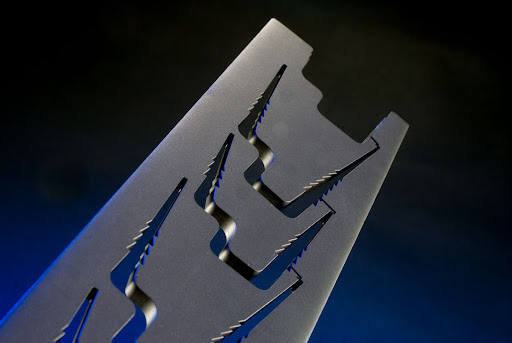
Our rapid prototyping services are second to none. Wire EDM technology allows us to machine parts quickly and easily out of medical-grade materials such as titanium and cobalt chrome without the need for custom fixtures and tooling, ensuring your products go to market faster and at a lower cost than the competition. In addition, the precision and accuracy of wire EDM is ideal for medical applications where complex geometries, tight tolerances and repeatability are essential.
Industries served:
- Orthopedics
- Cardiovascular
- Endoscopy
- Pharmaceutical
- Surgical instruments
- Implants and prosthetics
- Drug delivery
- General medical
Industry standards:
- AS 9100 certified.
- ISO 9001:2008 certified.
- ISO 13485:2016 certified.
- Current Good Manufacturing Practices (cGMP) Compliant
- Food and Drug Administration (FDA) Compliant
- Material usage protocols, such as the Restriction of Hazardous Materials (RoHS2) Directive, conflict mineral regulations (Dodd-Frank Wall Street Reform and Consumer Protection Act, Section 1502), etc.